🧪 11th Chemistry Important Questions 2025 – Public Exam Guide
📚 Ace Your Chemistry Public Exam with Important Questions!
Get the most important two-mark, three-mark, and five-mark questions for the 11th Chemistry Public Exam 2025, carefully selected from previous year board exam papers and important chapters. These questions will help you revise quickly and score high marks.
🔹 Chapter-wise 2-mark, 3-mark & 5-mark important questions
🔹 Based on Tamil Nadu Board syllabus
🔹 Covers Organic, Inorganic & Physical Chemistry
🔹 Includes high-weightage topics from past public exams

Two Marks
- Define – Mole?Ln1
- What do you understand by the term oxidation number?Ln1
- What is oxidation number. Write any three rules to find the oxidation number of an element?Ln1
- Define equivalent mass?Ln1
- Find emprical formula of fructose C6H12O6 and Caffeine C8H10N4O2? Ln1
- fructose C6H12O6 – CH6O
- Caffeine C8H10N4O2 – C4H5N2O
- Define avogadro number?Ln1 Pg7
- Define relative atomic mass?Ln1 Pg4
- What is the difference between molecular mass and molar mass? Calculate the molecular mass and molar mass for carbon monoxide.Ln1 37
- State pauli Exclusion principle? Ln2
- State ( n+l ) rule (Pg No : 51)
- State Hund’s rule (Pg No : 53)
- Define exchange energy (Pg No : 56)
- State Heisenberg’s uncertainty principle (Pg No : 42)
- How many orbitals are possible for n = 4?Ln2 (Pg No : 46)
- Define Principal quantum number?Ln2 (Pg No : 45)
- Define Azimuthal quantum number?Ln2 (Pg No : 45)
- Define Magnetic quantum number, spin quantum numberLn2 (Pg No : 46)
- Give the electronic confiuration of Mn2+ and Cr3+?Ln2 BB39
- Write the stable electronic configuration of cu and Cr?Ln2 Pg55
- State Modern periodic Law?Ln3
- Define electronegativity?Ln3
- What are isoelectronic ions? Give examples. ?Ln3 Pg25
- Explain the pauling method for the determination of ionic radius.?Ln3 39
- Ionisation potential of N is greater than that of O.Why?Ln3 BB44
- Define electron affinity?Ln3 pg86
- The element with atomic number 120 has not been discovered so far. What would be the IUPAC name and the symbol for this element? Predict the possible electronic configuration of this element.?Ch3 Evaluate Pg76
- What are isotopes? Write the names of isotopes of hydrogen.Ch4
- Explain the types of Hydrogen Bond with one example?Ch4 Pg113
- what is plaster of paris ?How is it prepared?Ln5
- Uses of plaster of Paris?Ln5 Pg148
- Give the colour of the following alkali metal in flame?Ln5 Pg128
- Name the metals which produce the following colours during flame test
- 1)Yellow 2)Lilac 3)Crimson red 4)Brick Red
- ?Ln5 Pg128
- Give the uses of calcium?Ln5 Pg141
- BeO is insoluble in water.Why ? Ln5 Pg142
- Write any two uses of sodium carbonate?Ln5 Pg133
- Write any two uses of sodium Bicarbonate?Ln5 Pg133
- Give the uses of Uses of Gypsum?Ch5 Pg146
- An alkali metal (x) forms a hydrated sulphate, X2 SO4 . 10H2 O. Is the metal more likely to be sodium (or) potassium.?Ln5
- Define Joule Thomson effect?Ln6
- State daltons law of partial pressure?Ln 6
- State boyle’s law?Ln6
- Would it be easier to drink water with a straw on the top of mount everest?Ln6 BB39
- State Gay – Lussac’s law?Ln6 Pg164
- State the various statements of second law of thermodynamics?Ln7 Pg210
- State zeroth law of thermodynamics?Ln7
- What is heat of combustion?Ln 7
- What is extensive properties? Give any two example?Ln 7
- What is Lattice energy?Ln7
- Define gibbs free energy?Ln7
- Write the third law of thermodynamics?Ln7 Pg217
- Define the heat of neutralization?Pg 206
- Define Hess’s Law?Ln7 Pgpg207
- Write a balanced chemical equation for the equilibrium reaction for which the equilibrium constant is given by expression Ln8 BB37
- State Le-Chatelier’s Principle?Ln 8
- State law of mass action?Ln8 Pg5
- State Henry’s law? Ln9
- What are the limitations of henry’s Law?Ln9
- State henry’s law and Raoult’s law?Ln9
- What is osmotic pressure?Ln9 Pg : 55
- Define the term isotonic solution?Ln9 Pg56
- Define mole fraction?Ln9 Pg33
- What are the properties of ideal solution?Ln9 pg46
- Draw the Lewis dot structures for Ammonia and Methane,H2O, HNO3? Ln10 Pg71
- State Lewis – Octate Rule?Ln10
- Define hybridisation?Ln10 Pg89
- Give the Iupac name of the following compounds?Ln11
- A.CH3 – O – CH3
- B.CH2 = CH – CH = CH2
- What are homologous series?Give example Ln11
- Write the function group for the following?Ln11
1.Aldehyde
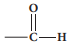
2.Ketone
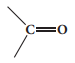
3.Carboxylic acid
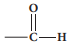
4.Ether
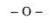
- Write short note on wurtz reaction?Ln 13
- How will you convert ethyl chloride in to i) ethane ii) n – butane? Ln13
- Write note on Friedel craft’s reaction ?Ln13Pg : 210
- How toluene is prepared by Wurtz-Fittig reaction?Ln13 Pg210
- Explain preparation of alkane from grignard reagent?Ln13 Pg185
- Preparation of aldehyde from Grignard Reagent?Ln13 Pg185
- Write two examples for ortho – para and meta directing groups?Ln13pg 216
- How will you convert ethyl chloride in to i) ethane ii) n – butane? / How will you prepare ethyle chloride from the following?Ln13 BB41
- How will you prepare phenol by Dow’s Process?Ln 14
- Write williamson’s Ether Synthesis?Ln14
- Complete the following reaction?Ln14 Pg 225
- What is particulate pollutant?Ln15
- Differentiate the following (i) BOD and COD (ii) Viable and non-viable particulate pollutants?Ln15
- What is green chemistry?Ln15 Pg274
- Define smog?Ln15 266
- What is greenhouse effect?Ln15 Pg263
- What is Global Warming? Ln15 Pg263
- How is acid rain formed? Ln15 pg264
- What would happen, if the greenhouse gases were totally missing in the earth’s atmosphere?Ln15 Q19
Three Marks
- What is limiting reagent?Ln1 Pg17
- Distinguish between oxidation and reduction?Ln1 Pg19
- What is disproportionate reaction?Give Example?Ln1 Pg28
- Caculate the oxidation number of the underlined element in the following species?
- a.H2SO4 b.CH2F2 c.CO2 d.H2O2
- Give the electronic configuration of Mn2+ and Cr3+?Ln2 39
- Derive De-Broglie equation?Ln2 Pg No 41
- What are quantum numbers?Ln2 (Pg No : 44)
- Define orbital? what are the n and l values for 3px and 4dx 2 -y 2 electron??Ln2 Bb32
- An atom of an element contains 35 electrons and 45 neutrons. Deduce ?Ln2 BB41
- Calculate the total number of angular nodes and radial nodes present in 3d and 4f orbitals. ?Ln2 evaluate Pg50
- calculate the effective nuclear charge of k+ ion?Ln3 Refer Guide
- Calculate the effective nuclear charge on 4s electron in scandium?Ln3
- Explain Diagonal relationship?Ln3 Pg90
- Predict the position of the element in periodic table satisfying the electronic configuration (n-1)d2, ns2 where n=5 ?Ch3 Evaluate Pg78
- Explain the periodic trend of ionisation potential?Ln3 BB40
- Explain the removal of temporary hardness of water by clarks method?Ln4 Pg109
- Write short note on ortho and para hydrogen?Ln4 Pg102
- How is tritium prepared.Give its half life period?Ln4 Pg104
- What are the uses of Hydrogen peroxide?Ln4 Pg112
- Write the uses of heavy water?Ln4 Pg111
- Mention the uses of deuterium.?Ln4 BB36
- Explain the exchange reactions of deuterium.?Ln4 BB34
- Why is beryllium different from other elements in that group?Ln5 Pg139
- Describe briefly the biological importance of magnesium?Ln5 Pg148
- Mention the uses of magnesium?Ln5 Pg148
- Uses of radium?Ln5 Pg142
- Describe briefly the biological importance of Calcium and magnesium?Ln5
- Describe briefly the biological importance of Sodium and Potassium?Ln5Pg135
- Give the systematic names for the following :(i) milk of magnesia (ii) lye etc BB31
- Explain the structure of Beryllium Chloride?Pg143
- Define Graham’s Law of diffusion and give the mathematical expression?Ln6 Pg168
- Derive ideal gas equation?Ln6 Pg 165
- Define inversion temperature?Ln6 Pg175
- Explain the application of dalton’s law?Ln6 Pg175
- What is diffusion and effusion?Ln6 Refer Guide
- What is entropy ?Ln7 Pg210
- Relationship between cp and cv?Ln7 pg201
- What are state and path functions?Give tow examples?Ln7 Pg190
- List out the difference between Intensive an Extensive property?Ln7 pg189
- Write the kelvin-plank statement?Ln7 Pg210
- List the characteristic’s of internal energy?Ln7 Pg191
- List out the applications of Bomb calorimeter?Ln7 Pg204
- Derive relation between enthalpy and internal energy / How will you measure
- ΔU and ΔH using calorimetry?Ln7 pg197
- Write down the Born-Haber cycle for the formation of CaCl2?Ln7 BB43
- Derive relationship between kp and kc?Ln8 Pg6
- Derive a general expression for the equilibrium constant KP and KC for the reaction 3H2 (g) + N2 (g) ⇌ 2NH3 (g)?Ln8 BB36
- What is the effect of added inert gas on the reaction at equilibrium at constant volume?Ch8 BB8
- What are colligative properties?Give example?Ln9 Refer Guide
- What is osmosis and osmotic pressure?Ln9 Pg55
- Calculate the molality of a solution containing 7.5g of glycine dissolved in 500g of water?Ln9 BB42
- What is dipole moment?Ln10 Pg79
- Explain Fajan’s rule?Ln10 Pg 81 3 – Points
- What is called resonance.Give example?Ln10 Pg78
- Define a)Bond Order B)Bond Length? Ln10 Pg76
- What type of hybridisations are possible in the following geometeries?Ln10 BB50
- Write the structural formula for the following compounds?Ln11 Pg122 Pg124
- Discuss cis – trans isomerism with suitable example?Ln11 Pg135
- Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.?Ln11 BB40
- Position isomerism?Ln11 Pg132
- What is inductive effect,Explain with example?Ln12 Pg166
- Two types of inductive effect?Ln12
- State and explain Markovnikoff’s rule with one example?Ln13 Pg194
- Using Huckel rule find out the compound Benzene whether it is aromatic or non aromatic?Ln13Pg206
- State Huckel’s rule?Ln13 Pg205
- What is polymerisation and how polyethylene is prepared?Ln13 pg199
- What happens when acetylene is passed through red hot tube?Ln13 Pg210
- Draw the different conformations of n-Butane?Ln13 Pg187
- Compare SN1 and SN2 reaction mechanisms. Ln14
- How will you prepare ethylene glycol from ethylene?Ln14
- Mention the uses of chloroform?Ln14 Pg238
- Carbylamine reaction?Ln14 Pg248
- Sandmeyer Reaction? Ln14 Pg242
- Write swarts reaction?Ln14 Pg231
- Write the uses of Freons?Ln14 Pg249
- 47. Write short notes on the the following i) Raschig process ii) Dows Process iii) Darzens process?Ln14 Pg242
- Harmful effects of chemical water pollutants:?Ln15 Pg271
- Which is considered to be earth’s protective umbrella? Why? ?Ln15 BB21
- What is air pollution?Ln15 pg261
- How will you control air pollution?Ln15 Pg261
Five Marks
- Calculate the molar of the following compounds?Ln1
- 1.Urea[NH2CONH2] 2-Acetone[CH3COCH3] 3.Boric Acid[H3BO3] 4.Ethanol[C2H5OH] 5.Sulphuric Acid [H2so4]
- What is Exchange Energy?Ln1 Pg56
- Calculate the emprical and molecular formula of a compound containing 76.6% carbon,6.38% hydrogen and rest oxygen its vapour density is 47.Ln1 BB42
- Balance the following equation by oxidation number method?All given in book ?Ln1 BB44
- An organic compound present in venegar has 40% carbon,6.6% hydrogen and 53.4 % oxygen.Find the Emprical formula of the compound?Ln 1 Pg11( Similar question from book)
- Explain combination redox reaction and disproportionation redox reaction?Ln1 22
- Explain Davison and Germer experiment?Ln2 Pg42
- State Aufbau Principle?Ln2 Pg52
- Write the expected electronic configuration and correct electronic configuration of chromium?Ln2 Pg 55
- Explain Bohr’s atomic model?Ln2 Pg39
- Postulates of bohr atom model?ln2 pg39
- List out the Limitations of Bohr’s atom Model?
- Write the time independent Scrodinger equation Expression?Ln2 Pg43
- Explain the variation of ionisation energy along a period and in a group?Ln3Pg85
- State the trends in the variation of electronegativity in group and periods. ?Ln3 BB47
- What are called inter and Intra Molecular hydrogen bonding give example?Ln 4 Pg114
- How para hydrogen is converted into ortho hydrogen?Ln4 BB35
- Write the different types of Hydrides with suitable example?Ln4 Pg113
- Why are alkali metals considered to be a powerful reducing agent?Ln4 Pg101
- How does alkali metal react with oxygen?Ln5 Pg130
- How does Lithium differ other elements in that group?Ln5 pg129
- Write the similarities between lithium and magnesium?Ln5 Pg129
- Explain the preparation of sodium carbonate by solvay process?Ln5 BB27
- List out the general characteristics of alkali metals?Ln5 Pg126
- Explain why Ca(OH)2 is used in white washing?Ln5 Pg145
- Define compressibility factor and give the equation?Ln6 Pg 169
- Derive the values of critical constants in terms of Vander waals constant?Ln6 Pg171
- Write the Van der Waals equation for a real gas. Explain the correction term for pressure and volume ? Ln6 BB40
- State Boyle’s Law?Ln6 Pg160
- What are ideal gases? In what way real gases differ from ideal gases.?Ln6BB29
- Derive relationship between ΔH and ΔU?Ln7 197
- State the various statements of second law of Thermodynamics?Ln7 Pg210
- Define reaction quotient?Ln7 Pg217
- Derive kp,kc values for dissociation of PCl5?Ln8 Pg13
- What is meant by Vant Hoff’s factor?Ln8 Pg20
- Explain the factors responsible for the deviation of solutions from Roults’s Law?Ln9 Pg48
- What is meant by elevation of boiling point and write its mathematical expression?Ln9 Pg51
- Mention the difference between ideal and non – Ideal solution?Ln9 Pg45
- What are ideal solutions?Ln9 Pg45
- State Raoult law and obtain expression for lowering of vapour pressure when nonvolatile solute is dissolved in solvent.?Ln9 BB34
- Factors / Reasons responsible for deviation from Raoult’s law?Ln9 Pg48
- Which bond is stronger sigma bond or Pi bond ? Ln10 BB43
- What are salient features of VB theory?Ln10 Pg86
- Draw the Lewis dot structures for Ammonia and Methane? Ln10 Pg71
- Draw the Lewis dot structures? Ln10 BB41
- Draw MO diagram for H2 and other molecules Molecule and calculate its Bond order?Ln10 Pg99
- What are the salient features of Molecular Orbital theory?Ln10 Pg97
- Discuss the formation of N2 / O2 molecules using MO Theory?Ln10 BB38
- Define Ionic Bond,Covalent Bond,Co-ordinate covalent bond?Ln10 Pg69
- Explain VSEPR theory. Applying this theory to predict the shapes of IF7, and SF6?Ln10 BB51
- Give brief description of the principles of 1.Fractional DistillationLn11 Pg150
- 2.Column Chromatography/ Paper Chromatography / Thin Layer chromatographyLn11 Pg152
- Write the characteristics of organic compounds?Ln11 Pg111?Ln11 Pg132
- Explain metamerism?Ln11 Pg133
- Write functional isomerism with an example?Ln 11 Pg133
- Find the functional group of the following compounds?Ln11
- A.Acetaldehyde B.Oxalic acid C.Dimethyl ether D.Methylamine
- Describe the classification of organic compounds based on their structure?Ln11 112
- What is sublimation?Give Example?Ln11 Pg149
- What are the condition for optical activity?Ln11 Pg137
- What is Rf Value?Ln11 Pg153
- Write Short notes on 1.Nucleophiles and electrophilesGive two examples Ln12 Pg164 2.Mesomeric EffectLn12 Pg 168
- Explain electromeric effect?Ln12
- Write the differences between electrophiles and Nucleophiles?Ln12Pg164
- Short note – Hyper Conjugation?Ln12 Pg170
- How does Huckel rule help to decide te aromatic character of a compound?Ln13 BB37
- Describe the mechanism of Nitration of benzene.?Ln13 BB36
- Suggest a simple chemical test to distinguish propane and propene?Ln13 BB39
- Explain the structure of Benzene?Ln13 Pg207
- Explain sulphonation reaction of benzene?Ln13 Pg211
- Write short note on 1)Ozonolysis 2)Plymerisation 3) Birch Reduction ? Ln13 Pg198
- Explain mechanism of Sn1 Reaction?Ln14 BB47
- Write the structures of the following compound?Ln14 Pg 229 Refer Guide
- 1.1-Bromo-4-ethyl cyclohexane, 2.1,4-Dichlorobut-2-ene
- A.acetaldehyde b.Tertiary-butyl iodide c.3-chlorobutanal
- Explain E2 Mechanism?Ln14 Pg237
- How DDT prepared.Give its uses?Ln14 Pg250
- Starting from ch3MgI, how will you prepare the following?Ln14BB48
- A.Ethylalcohol B.Acetaldehyde C.Ethyl Methyl Ether?
- What is Eutrophication?Ln15 Pg271
🎯 Why Focus on Important Questions for 11th Chemistry?
The 11th Chemistry public exam consists of short-answer (two marks), medium-answer (three marks), and long-answer (five marks) questions. Focusing on these helps students maximize their scores efficiently.
📚 Chapter-wise Important Questions Covered:
🔹 Organic Chemistry – Hydrocarbons, Alcohols, Aldehydes, Ketones, Carboxylic Acids
🔹 Inorganic Chemistry – P-Block Elements, Coordination Compounds, Metallurgy
🔹 Physical Chemistry – Thermodynamics, Electrochemistry, Chemical Kinetics
🔹 Solid State, Solutions & Surface Chemistry
📢 More 12th Physics Exam Resources:
📘 12th physics important two marks public exam 2025
📗 12th physics important three marks public exam 2025
📚 12th physics important five marks public exam 2025
📢 External References for Chemistry Exam Preparation:
🔗 11th Chemistry One Marks shortcuts
🔗 11th Chemistry Public Exam study plan 2025
🚀 Maximize Your Scores! Visit Padidaa.com for the latest 12th Public Exam updates, study materials, and video lectures! 🎯
